COVID-19 Antigen Test Kits
COVID-19 Antigen Rapid Test Principle
The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid (N) protein in upper respiratory samples (nasal swabs). This lateral flow assay is designed with the sandwich immunoassay format. When the specimen is added onto the sample pad of a test cassette, coronavirus N protein binds with colloidal gold-labeled SARS-CoV-2 N protein antibody to form an antibody-antigen (Ab-Ag) complex. The Ab-Ag complex is captured by SARS-CoV-2 N protein antibody (Rabbit monoclonal antibody) when migrating to the test line under capillary action. A red-colored band will appear on the test line, which indicates the specimen is COVID-19 nucleocapsid protein positive. No color band will appear on the test line if the specimen does not contain any coronavirus antigen (N protein), or the antigen level is below detection limit.
Coronavirus Antigen (Ag) Rapid Test Kit PrincipleCoronavirus Antigen (Ag) Rapid Test Kit Principle
The conjugation pad also contains colloidal gold-labeled chicken IgY, which is captured by Goat anti-chicken IgY on the control line as procedural control. A colored band on the control line represents the proper liquid flow through the cassette; the absence of a colored band on the control line indicates insufficient sample or buffer volume.
| Product name | COVID-19 Antigen Test Kits |
| Material | Medical grade plastic |
| Logo printing | Available |
| Usage | Medical using, hospital using, home nursing, etc |
| Certificate | CE, FDA510(K) |
| Sample | One Sample free |
| Stock | Yes |
| OEM/ODM | Available |
| Storage | 2-30'C |
| Payment | TT L/C |

Product Components


Operation procedure

Performance

Order information

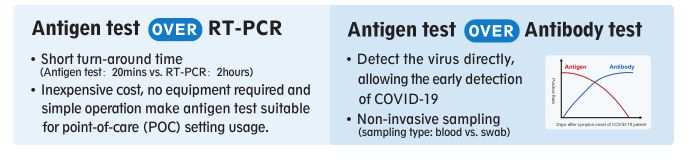
CURRENT DIAGNOSTIC METHODS FOR COVID-19


ANTIGEN TEST ADVANTAGES
ANTIGEN TEST APPLICATION
Similar to RT-PCR, the detection of antigen indicates the active infection. Under
the circumstance that the area(s) is still undergoing widespread community
transmission and/or the area with limited RT-PCR resources, antigen can be
used for aiding in the diagnosis of COVID-19 suspect patients.
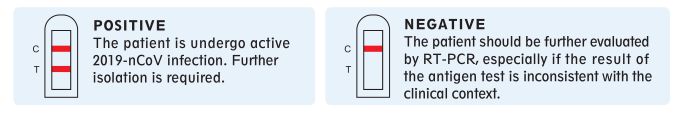
Result interpretation
The Production Workshop
FAQ
Q1: what's your minimum order quantity?
A: It depends on what product it is. Please contact me for details.
Q2: Do you support OEM/ODM?
A: YES, we can prototype and customize the ideal product according to your specific application.
Q3: Do you have any certificates?
A: YES, we have all the certificates required for export and local import custom clearance.
Q4: Where is your main market located?
A: 60% in American Countries, 30% in European Countries and 10% in Middle East.
Q5: What's your lead time?
A: For mass production, we deliver by batches and first batch can be delivered within 7 days.