High Quality Covid-19 Vaccine (Vero Cell) - Sinopharm (Beijing): BBIBP-CorV – Jinlian
High Quality Covid-19 Vaccine (Vero Cell) - Sinopharm (Beijing): BBIBP-CorV – Jinlian Detail:
Sinopharm BBIBP-CorV COVID-19 is an inactivated vaccine made from culture-grown virus particles that lack pathogenic ability. This vaccine candidate was developed by Sinopharm Holdings and the Beijing Institute of Biological Products.
The Sinopharm BBIBP-CorV vaccine works by allowing the immune system to produce antibodies against SARS-CoV-2 beta coronavirus. Inactivated virus vaccines have been used for decades, such as rabies vaccine and hepatitis A vaccine. This development technology has been successfully applied to many well-known vaccines, such as the rabies vaccine.
Sinopharm’s SARS-CoV-2 strain (WIV04 strain and library number MN996528) was isolated from a patient at Jinyintan Hospital in Wuhan, China. The virus was propagated in culture in a competent Vero cell line, and the supernatant of infected cells was inactivated with β-propiolactone (1:4000 vol/vol, 2 to 8°C) for 48 hours. After clarification of cell debris and ultrafiltration, a second β-propiolactone inactivation was performed under the same conditions as the first inactivation. According to WHO, the vaccine was adsorbed onto 0.5 mg of alum and loaded into prefilled syringes in 0.5 mL of sterile phosphate-buffered saline without preservatives.
On December 31, 2020, the State Drug Administration announced the approval of the experimental vaccine developed by Sinopharm.
On May 7, 2021, the World Health Organization announced the approval of the vaccine. WHO’s emergency use list enabled countries to expedite their own regulatory approvals to import and administer COVID-19 vaccine. The WHO Advisory Expert Group on Immunization Strategies has also completed its review of the vaccine. Based on all available evidence, WHO recommends two doses of vaccine, three to four weeks apart, for adults 18 years and older. Vaccine efficacy against symptomatic and hospitalized disease is estimated at 79% for all age groups combined.
The American Medical Association published “A Randomized Clinical Trial: Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults” on May 26, 2021, concluding that “in this prespecified interim analysis of a randomized clinical trial, adults The 2 inactivated SARS-CoV-2 vaccines administered in this prespecified interim analysis of randomized clinical trials significantly reduced the risk of symptomatic COVID-19, and serious adverse events were rare.” In this phase 3 randomized trial in adults, the efficacy of the 2 inactivated whole virus vaccines in symptomatic COVID-19 cases was 72.8% and 78.1%, respectively. 2 vaccines had rare serious adverse events with similar frequency to the alum-only control group, and most were unrelated to vaccination. An exploratory analysis found that the 2 vaccines induced measurable neutralizing antibodies, similar to the results of the phase 1/2 trial.
The WHO SAGE Working Group published a review of the Sinopharm/BBIBP COVID-19 vaccine on May 10, 2021. GAVI’s COVID-19 vaccine incorporates a vaccine vial monitor that tells health workers whether the vaccine has been stored properly and has not been exposed to overheating. As a result, damage, GAVI reported on May 14, 2021. smart labels manufactured by Zebra Technologies and made by Temptime Corporation, consist of a circle with a lighter colored square in the middle, made of a colorless chemical that irreversibly develops color over time. This becomes darker to give a visual indication of cumulative heat exposure. Once the vial has been exposed to heat beyond its optimal storage range, the square becomes darker than the circle, indicating that the vaccine should no longer be used.
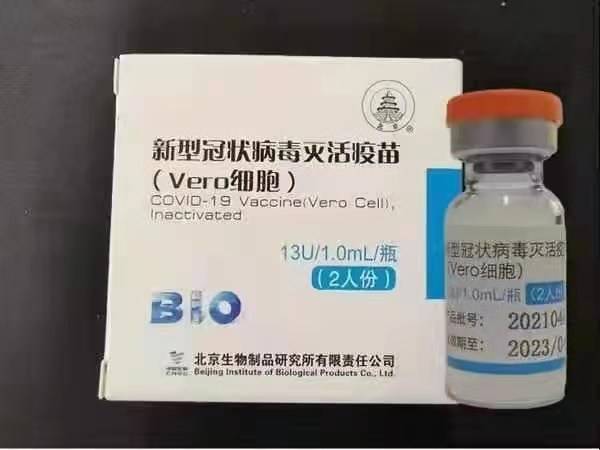
National Drug BBIBP-CorV COVID-19 vaccine drug library registration number: DB15807.
Product detail pictures:
Related Product Guide:
"Based on domestic market and expand abroad business" is our enhancement strategy for High Quality Covid-19 Vaccine (Vero Cell) - Sinopharm (Beijing): BBIBP-CorV – Jinlian , The product will supply to all over the world, such as: Curacao, Armenia, Japan, Strong infrastructure is the need of any organization. We are backed with a robust infrastructural facility that enables us to manufacture, store, quality check and dispatch our products worldwide. To maintain smooth work flow, we have sectioned our infrastructure into a number of departments. All these departments are functional with latest tools, modernized machines and equipment. Owing to which, we are able to accomplish voluminous production without compromising upon the quality.
Problems can be quickly and effectively resolved, it is worth to be trust and working together.